Discover what CRYSVITA can do in children and adults with XLH
Learn more about patients who might benefit from CRYSVITA
Patients with XLH may need targeted therapy to address the underlying causes of chronic hypophosphatemia in XLH. In your practice, you may know these types of patients.
Pediatric patient profile
4-year-old male with spontaneous XLH


Pediatric patient profile
Liam is a 4-year-old boy who loves to run around and play. He presented with symptoms at 20 months and underwent several specialty evaluations before being diagnosed with spontaneous XLH at 25 months.*
*This case is adapted from a real patient and is intended for illustrative purposes only, not as recommendations of care or management. XLH=X-linked hypophosphatemia.
 Show description
Show description
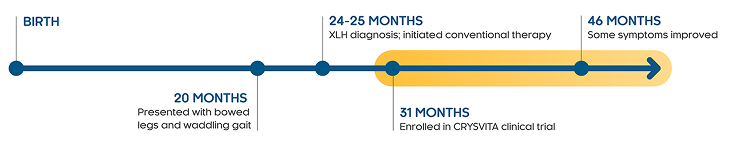
Timeline of events for Liam starting from birth to symptom improvement. At 20 months, Liam presented with bowed legs and waddling gait. At 24-25 months, he was diagnosed with XLH and initiated on conventional therapy. At 31 months, he was enrolled in the CRYSVITA clinical trial, and by 46 months, he had some improvement in symptoms.
Medical history
- 20-month-old male presented with bowed legs
- Bowing present since birth, worsened once patient started walking
- Other physical exam findings: waddling gait, cannot run well
- Suspected diagnosis: congenital bilateral knee bowing
- Exam at 24 months: worsened bowing of legs and waddling gait noted
- X-ray findings: bilateral bowing of femur and tibia; cupping of distal femur, proximal and distal tibia and fibula
- Suspected diagnosis: rickets
Evaluation at academic center
- Physical exam at 24.5 months: severe knee bowing, intercondylar distance of 5 cm, short stature, height in 1st percentile
- X-rays: metaphyseal flaring at wrists and ankles
- Laboratory findings (Table below)
- Suspected diagnosis: hypophosphatemic rickets
- Upon genetic evaluation at 25 months, pathologic PHEX variant identified; mother negative
| Test
(reference range† unit) |
Early evaluation
(24.5 months) |
Prior to CRYSVITA
(31 months) |
|---|---|---|
| Serum phosphorus (3.2-6.1 mg/dL) |
2.9 | 2.9 |
| TmP/GFR‡ (3.2-6.1 mg/dL) |
n/a | 2.8 |
| 25(OH)D (20-50 ng/dL) |
26 | 24 |
| ALP (ULRR for 1-15 y: 297-385 U/L) |
636 | 459 |
| Serum calcium (0-2 y: 9.0-11.0 mg/dL; 2-18 y: 8.4-10.3 mg/dL) |
9.4 | 9.5 |
| PTH (14-72 pg/mL) |
56 | 20 |
| Test
(reference range† unit) Serum phosphorus (3.2-6.1 mg/dL) |
|---|
|
Early evaluation (24.5 months) |
| 2.9 |
|
Prior to CRYSVITA (31 months) |
| 2.9 |
| TmP/GFR‡ (>1 year: 2.6-4.4 mg/dL) |
|
Early evaluation (24.5 months) |
| n/a |
|
Prior to CRYSVITA (31 months) |
| 2.8 |
| 25(OH)D (20-50 ng/dL) |
|
Early evaluation (24.5 months) |
| 26 |
|
Prior to CRYSVITA (31 months) |
| 24 |
| ALP (ULRR for 1-15 y: 297-385 U/L) |
|
Early evaluation (24.5 months) |
| 636 |
|
Prior to CRYSVITA (31 months) |
| 459 |
| Serum calcium (0-2 y: 9.0-11.0 mg/dL; 2-18 y: 8.4-10.3 mg/dL) |
|
Early evaluation (24.5 months) |
| 9.4 |
|
Prior to CRYSVITA (31 months) |
| 9.5 |
| PTH (14-72 pg/mL) |
|
Early evaluation (24.5 months) |
| 56 |
|
Prior to CRYSVITA (31 months) |
| 20 |
†Indicates normal range, age, and gender matched. Reference range values in this table were based on parameters in a phase 3 study of CRYSVITA in pediatric patients, as well as values presented in Dahir et al.1
‡Target range.
25(OH)D=25-hydroxyvitamin D (calcifediol); ALP=alkaline phosphatase; PTH=parathyroid hormone; TmP/GFR=ratio of tubular maximum reabsorption of phosphorus to glomerular filtration rate; ULRR=upper limit of reference range.
Diagnosis and initial treatment
- Spontaneous XLH
- Treatment: oral calcitriol and phosphate
Diagnosis progression
Evaluation at academic center
- Physical exam at 29 months: femoral bowing; obvious genu varus—knees 4.5 cm apart, ankles touching; bilateral tibial bowing
- Height 5th percentile
- X-rays: metaphyseal widening at wrists, mild cupping of distal radius and ulna
- Knees: marked bowing, cupping and fraying of growth plates
- Laboratory findings (Table above)
- Enrolled in CRYSVITA clinical trial at 31 months
See Liam’s detailed case study and the outcome of CRYSVITA treatment here.
DownloadDiscover how CRYSVITA helped pediatric patients like Liam in a clinical trial.
Reference:
- 1. Dahir K, et al. X-Linked Hypophosphatemia: A New Era in Management. J Endocr Soc. 2020;4(12):bvaa151.

Adult patient profile
37-year-old female with inherited XLH

Adult patient profile
Nicole is a 37-year-old mother, whose young child keeps her on her toes and busy. She was diagnosed with XLH as a toddler and suffered from XLH-associated symptoms throughout adulthood, including fractures, joint stiffness, pain, and diminished physical function.*
*This case is adapted from a real patient and is intended for illustrative purposes only, not as recommendations of care or management. XLH=X-linked hypophosphatemia.

 Show description
Show description
Timeline of events for Nicole starting from birth to symptom improvement. At birth, no known abnormalities were detected for Nicole. At 2 years of age, she was diagnosed with XLH, which was later confirmed by genetic testing when she was 18 years old. By 35 years of age, Nicole began experiencing joint stiffness in her hips, knees, and ankles. When she was 36 years old, CRYSVITA therapy was initiated and she began to experience some symptom improvement by 37 years of age.
Medical history
- Age 2: Diagnosed with XLH
- Initiated oral phosphate and calcitriol and was compliant
- Age 13: XLH diagnosis was confirmed by genetic testing
XLH symptoms and associated complications in adulthood
- Chiari malformation requiring 2 corrective surgeries
- Chronic ankle pain and swelling in joints
- Gait abnormalities
- Sustained pelvic fracture during childbirth
- Age 33: discontinued oral phosphate and calcitriol
Evaluation prior to CRYSVITA
- Age 35: presented to adult endocrinology with bowing of upper and lower extremities and joint stiffness in hips, knees, and ankles
- Height 5th percentile
- Physical exam
- Height, 5’2”
- Required the use of assistive walking device (cane) for long distances and handicap tags due to mobility challenges
- Tinnitus in right ear
- Calcifications noted on prior renal ultrasound
- Laboratory findings (Table below)
- Physical therapy evaluation
- Required assistance to rise from seated to standing position
- Tight hip flexors; limited range of motion in hips
- X-rays
- Prominent enthesophytes associated with the calcaneus bilaterally as well as ankle and midfoot arthritis; slight bowing seen bilaterally in lower legs
- Hypertrophic bone formation occurring at the hip articulation and trochanters; sclerosis at the sacroiliac joints; slight bilateral bowing of femurs
- Bowing deformity at each femur and degenerative changes of the knees including bilateral articular surface irregularities of the femoral condyles
- Bilateral bowing of femurs, tibias, and fibulas; bones diffusely demineralized and bilateral narrowing of joint space compartment also noted
| Test
(reference range† unit) |
Early evaluation
(24.5 months) |
|---|---|
| Serum phosphorus (2.5-4.5 mg/dL) |
1.6 |
| 1,25(OH)2D (18-72 pg/mL) |
40.3 |
| 25(OH)D (20-50 ng/mL) |
35 |
| BSAP (2.9-14.5 µg/L) |
25 |
| PTH (14-72 pg/mL) |
55 |
| Creatinine (0.50-1.10 mg/dL) |
0.74 |
| Test
(reference range† unit) Serum phosphorus (2.5-4.5 mg/dL) |
|---|
|
Early evaluation (35 years) |
| 1.6 |
| 1,25(OH)2D (18-72 pg/mL) |
|
Early evaluation (35 years) |
| 40.3 |
| 25(OH)D (20-50 ng/mL) |
|
Early evaluation (35 years) |
| 35 |
| BSAP (2.9-14.5 µg/L) |
|
Early evaluation (35 years) |
| 25 |
| PTH (14-72 pg/mL) |
|
Early evaluation (35 years) |
| 55 |
| Creatinine (0.50-1.10 mg/dL) |
|
Early evaluation (35 years) |
| 0.74 |
†Indicates normal range, age, and gender matched. Note that normal range values may vary depending on reference dataset. Reference range values in this table were based on parameters in the clinical studies of CRYSVITA in adult patients with XLH, as well as values presented in Dahir et al.1
1,25(OH)2D=1,25 dihydroxyvitamin D; 25(OH)D=25-hydroxyvitamin D (calcifediol); BSAP=bone-specific alkaline phosphatase, also known as BAP; PTH=parathyroid hormone.
Diagnosis and initial treatment
- XLH diagnosis reconfirmed
- Treatment: CRYSVITA
See Nicole’s detailed case study and the outcome of CRYSVITA treatment here.
DownloadDiscover how CRYSVITA CRYSVITA helped adult patients like Nicole in clinical trials.
Reference:
- 1. Dahir K, et al. X-Linked Hypophosphatemia: A New Era in Management. J Endocr Soc. 2020;4(12):bvaa151.



