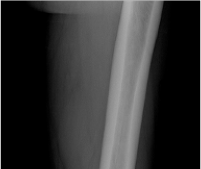
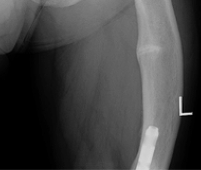
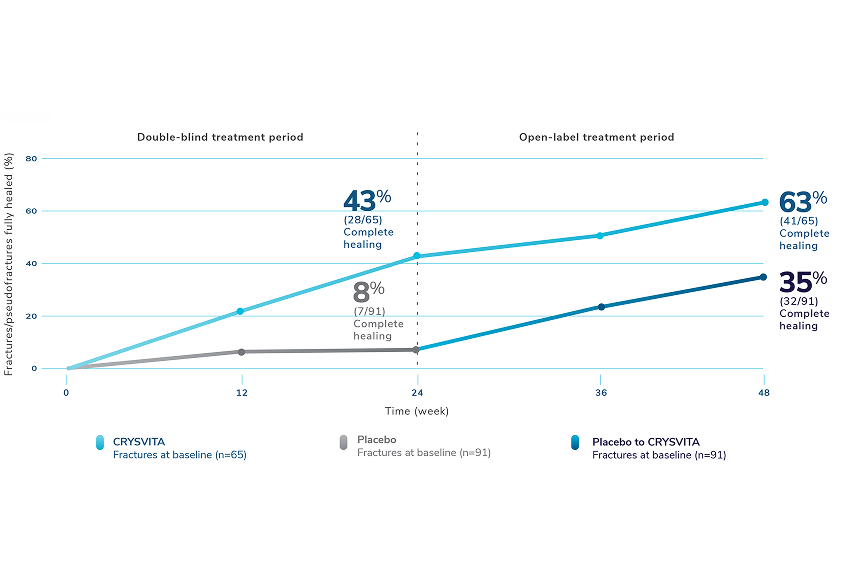
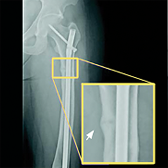
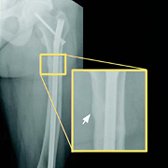
CRYSVITA® (burosumab-twza) is a fibroblast growth factor 23 (FGF23) blocking antibody indicated for the treatment of X-linked hypophosphatemia (XLH) in adult and pediatric patients 6 months of age and older.
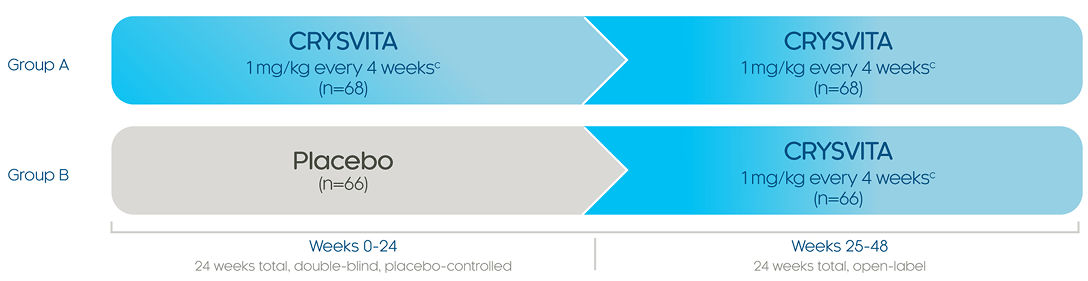
Efficacy & safety in adults
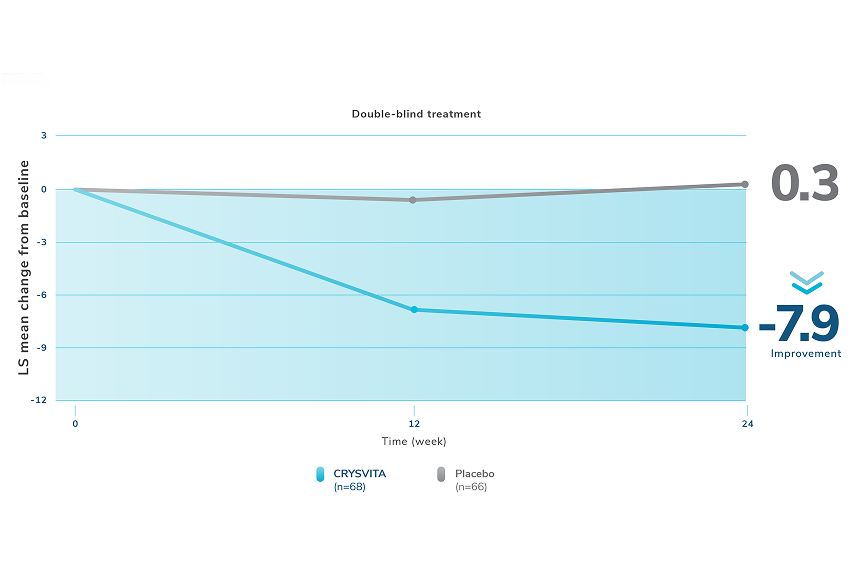
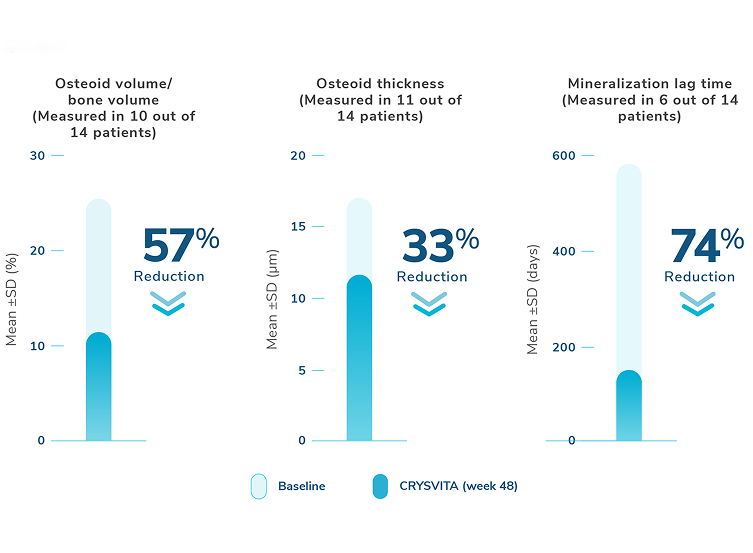
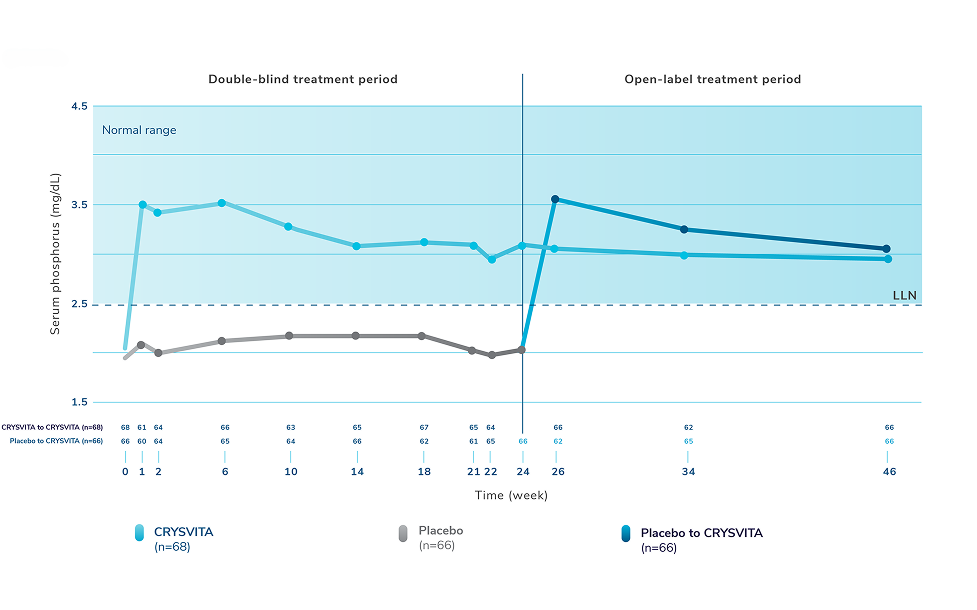
Healing of fractures was assessed at week 24 and healing of osteomalacia was assessed at
week 48 in adult patients.
Meet Aly, a real CRYSVITA patient







 Show description
Show description